Abstract
Over the past decade Nanoparticle Tracking Analysis (NTA) has emerged as a rapid, essential characterization tool for Extracellular Vesicle and Exosome research (Konoshenko et al., 2018, Giebel & Helmbrecht, 2017, Soo et al., 2012). In combination with fluorescence detection (f-NTA) the reliable and robust technology enables the user to identify dedicated Exosome populations as well as phenotyping Extracellular Vesicles with specific biomarkers (Rahbari et al., 2019). Here we report a unique method to identify platelet-derived Exosomes from plasma samples using specific fluorescently labelled CD41 antibody and the Particle Metrix ZetaView® instrument.
Introduction
Exosomes are small sized membrane surrounded Extracellular Vesicles (EV). They were first discovered in the maturing mammalian reticulocytes (Johnstone et al., 1987). However, the secretion of Exosomes has been shown in almost all biological cells (Raposo et al. 2013, Yanez-Mo et al. 2015). Even in plants (Stanly et al., 2016) and bacterial systems (Deatherage & Cookson, 2012) the existence of Exosomes has been described. Among others, the vesicles play an important role in:
- Maintaining the homeostasis (Baixauli et al. 2014)
- Cell-to-cell communication (Mathivanan et al. 2010)
- Tumor metastasis (Becker et al. 2016)
- Inflammatory processes (Console et al. 2019)
- other pathophysiological activities (Record et al. 2014).
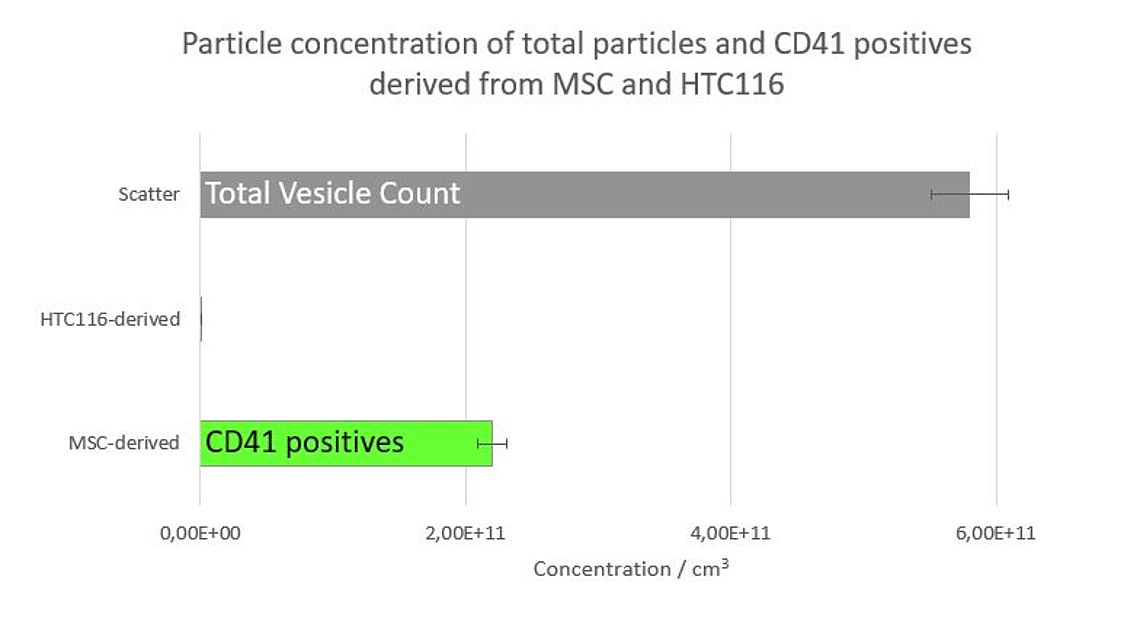
Figure 1: Particle concentration of total particles (100%) (grey) and CD41 positives derived from MSC (41,4%) and HTC116 (0%).
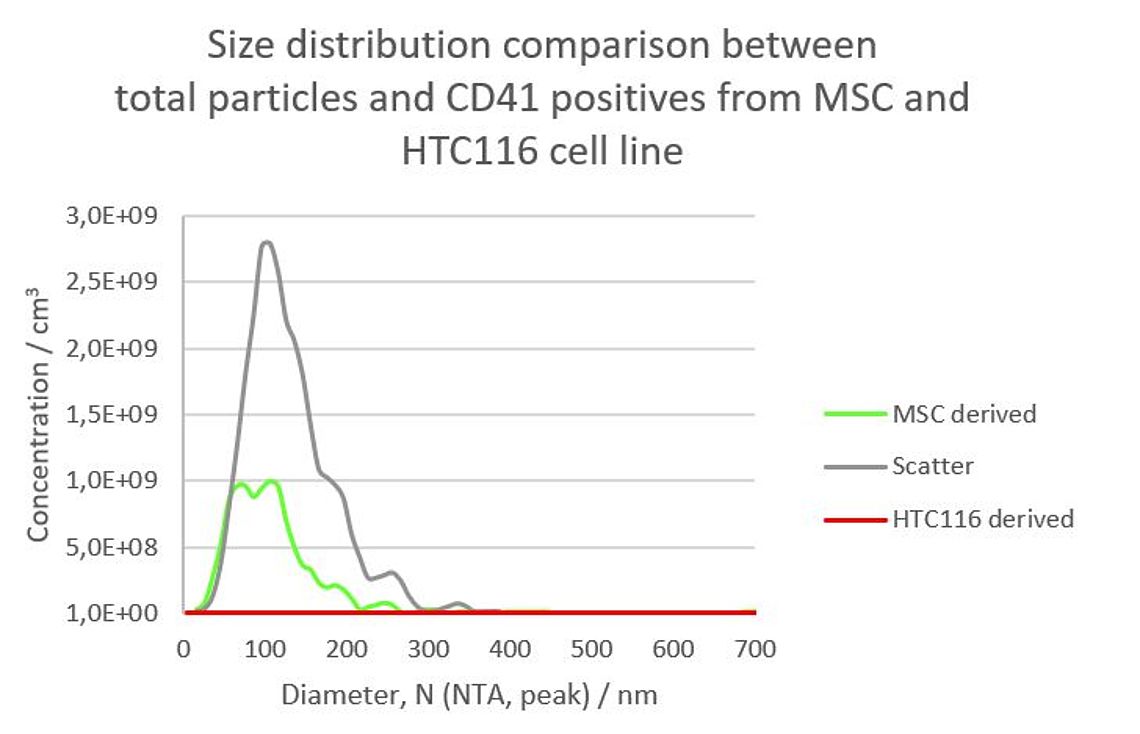
Figure 2: Size distribution of an EV preparation from a HCT cell line in scatter mode (gray) and stained with Cell Mask© Deep Red (red).
Due to the important functions and the almost ubiquitous presence of Extracellular Vesicles they are currently investigated in many laboratories worldwide. Among other technologies like Western Blotting, Elisa or Electron microscopy, Nanoparticle Tracking Analysis (NTA) is a widely used and state-of-the-art technology to characterize EVs (Rupert et al., 2017). Unfortunately, the NTA scatter technology alone is not powerful enough to discriminate between different subsets of Exosomes.
A new, fast and reliable method to specifically stain and measure platelet-derived Exosomes with fluorescently labelled CD41 antibody and the Particle Metrix ZetaView® Instrument (Meerbusch, Germany) is reported. The unique scanning technology of the instrument combines very short acquisition times of 0.5 to 1 seconds to limit fluorescence bleaching with statistically relevant volumes to ensure reliable data.
Methods
9 µl of an MSC derived EVs or EVs from HCT116 cell line (HansaBioMed, Estonia) were thoroughly mixed with 1 µg of a CD41-Alexa488 monoclonal antibody (Clone MEM06, Thermo Fisher, USA) and incubated for 2 hours at room temperature in the dark. The staining mix was then diluted 1:1.000 with particulate free 1 X PBS (Thermo Fisher, USA) to accomplish a total vesicle concentration of about 5.0 x 107 particles/ml. The sample was measured for size and concentration in scatter as well as in fluorescence mode with a 488 nm excitation laser on a Particle Metrix ZetaView QUATT® and the ZetaView® software version 8.05.10 with anti-bleach technology (Particle Metrix, Germany). The percentage of platelet derived, CD41 positive Exosomes was calculated by dividing the concentration in fluorescence mode by the total particle count in scatter mode.
Results & Conclusions
The well-established and widely used scatter based NTA technology is unfortunately not able to discriminate between different subpopulations within a heterogeneous EV sample. This challenging task needs more specific technologies like the fluorescence-based f-NTA (Rahbari et al., 2019, Weber et al., 2019). Here we describe a fast and easy technique to analyse platelet-derived subpopulations within different EV samples using a specific monoclonal CD41 antibody. The stained samples were analysed for size and concentration in scatter mode (total particle count) as well as in fluorescence mode with a blue excitation laser (λ = 488 nm) and a green emission filter set.
The data clearly show that the MSC sample contains about 41.4% CD41 positive platelet-derived Exosomes whereas no CD41 positive EVs could be detected in the preparation from the HTC116 cell line (Figure 1). Simultaneously performed size measurements showed that the CD41 positive Exosomes have a considerable smaller mean diameter (approx. 90 nm) than the total particles (114 nm) (Figure 2). A very likely explanation for this discrepancy is that bigger non-vesicular particles (e.g. nanobubbles, salt principates or protein aggregates) in the scatter measurement shift the size distribution to a bigger size. The double peak in the size distribution of the CD41 positive EVs derived from MSC cells (Figure 2, green curve), however could not be explained so far and needs further investigation.
Taken together, these results show, that fluorescent NTA (f-NTA) with the anti-CD41 antibody can detect platelet derived Exosomes in a diverse EV preparation. Moreover, recently published data with anti-Vimentin, anti-LAMP1 (Weber at al., 2019), anti-CD63 or anti-TSG101 (Rahbari et al., 2019) indicate a much broader utility of f-NTA in EV subpopulation detection.
References
Baixauli F., Lopez-Otin C., Mittelbrunn M. Exosomes and autophagy: coordinated mechanisms for the maintenance of cellular fitness. Front Immunol. 2014; 5:403.
Becker A., Thakur B., Weiss J. M., Kim H. S., Peinado H., Lyden D. Extracellular Vesicles in Cancer: Cell-to-Cell Mediators of Metastasis. Cancer Cell. 2016 Dec 12;30(6):836-848.
Console L., Scalise M., Indiveri C. Exosomes in inflammation and role as biomarkers. Clin Chim Acta. 2019; 488:165-171.
Deatherage B.L. and Cookson B.T. Membrane Vesicle Release in Bacteria, Eukaryotes, and Archaea: a Conserved yet Underappreciated Aspect of Microbial Life. Infect Immun. 2012; 80(6):1948-57.
Giebel B, Helmbrecht C. Methods to Analyze EVs; Methods Mol Biol. 2017;1545:1-20.
Konoshenko M.Y., Lekchnov E.A., Vlassov A.V., Laktionov P.P. Isolation of Extracellular Vesicles: General Methodologies and Latest Trends. Biomed Res Int. 2018: 8545347.
Johnstone R. M., Adam M., Hammond J. R., Orr L., Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). The Journal of Biological Chemistry. 1987; 262 (19): 9412–20
Mathivanan S., Ji H., Simpson R.J. Exosomes: extracellular organelles important in intercellular communication. J Proteom. 2010;73(10):1907–1920.
Rahbari M., Pecqueux m:, Aust D., Stephan H., Tiebel O., Chatzigeorgiou A., Tonn T., Baenke B., Rao V., Ziegler N., Greif H., Lin K., Weitz J., Rahbari N. N. and Kahlert C. Expression of Glypican 3 Is an IndependentPrognostic Biomarker in Primary Gastro-Esophageal Adenocarcinoma and Corresponding Serum Exosomes. J Clin Med. 2019 May 16;8(5).
Raposo G., Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200(4):373–383.
Record M., Carayon K., Poirot M., Silvente-Poirot S. Exosomes as new vesicular lipid transporters involved in cell-cell communication and various pathophysiologies. Biochim Biophys Acta. 2014;1841(1):108–120.
Rupert, D. L. M., Claudio, V. Lässer, C. Bally, M. Methods for the physical characterization and quantification of extracellular vesicles in biological samples. Biochim Biophys Acta Gen Subj. 2017; 1861(1 Pt A):3164-3179.
Soo C.Y., Song Y., Zheng Y., Campbell E.C., Riches A.C., Gunn-Moore F. and Powis S.J. Nanoparticle tracking analysis monitors microvesicle and exosome secretion from immune cells. Immunology. 2012, 136:192–197.
Stanly C., Fiume I., Capasso G., Pocsfalvi G. Isolation of Exosome-Like Vesicles from Plants by Ultracentrifugation on Sucrose/Deuterium Oxide (D2O) Density Cushions. Methods Mol Biol. 2016; 1459:259-69.
Weber A., Wehmeyer J. C., Schmidt V., Lichtenberg A., Akhyari P. Rapid Fluorescence-based Characterization of Single ExtracellularVesicles in Human Blood with Nanoparticle-tracking Analysis. J. Vis. Exp. 2019 (143), e58731
Yanez-Mo M., Siljander P.R., Andreu Z., Zavec A.B., Borras F.E., Buzas E.I., Buzas K., Casal E., Cappello F., Carvalho J., Colas E., Cordeiro-da Silva A., Fais S., Falcon-Perez J.M., Ghobrial I.M., Giebel B., Gimona M., Graner M., Gursel I., Gursel M., Heegaard N.H., Hendrix A., Kierulf P., Kokubun K., Kosanovic M., Kralj-Iglic V., Kramer-Albers E.M., Laitinen S., Lasser C., Lener T., Ligeti E., Line A., Lipps G., Llorente A., Lotvall J., Mancek-Keber M., Marcilla A., Mittelbrunn M., Nazarenko I., Nolte-’t Hoen E.N., Nyman T.A., O’Driscoll L., Olivan M., Oliveira C., Pallinger E., Del Portillo H.A., Reventos J., Rigau M., Rohde E., Sammar M., Sanchez-Madrid F., Santarem N., Schallmoser K., Ostenfeld M.S., Stoorvogel W., Stukelj R., Van der Grein S.G., Vasconcelos M.H., Wauben M.H., De Wever O. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015; 4:27066.
Acknowledgements
We thank Michel Bremer and Bernd Giebel, (University Hospital Essen, Germany) for the kind preparation and supply of MSC-EVs.
